“I just think a lot of this comes down to communication. It's just, I think there are times we lack the right kind of communication.”
Study Manager, Top 10 Pharma
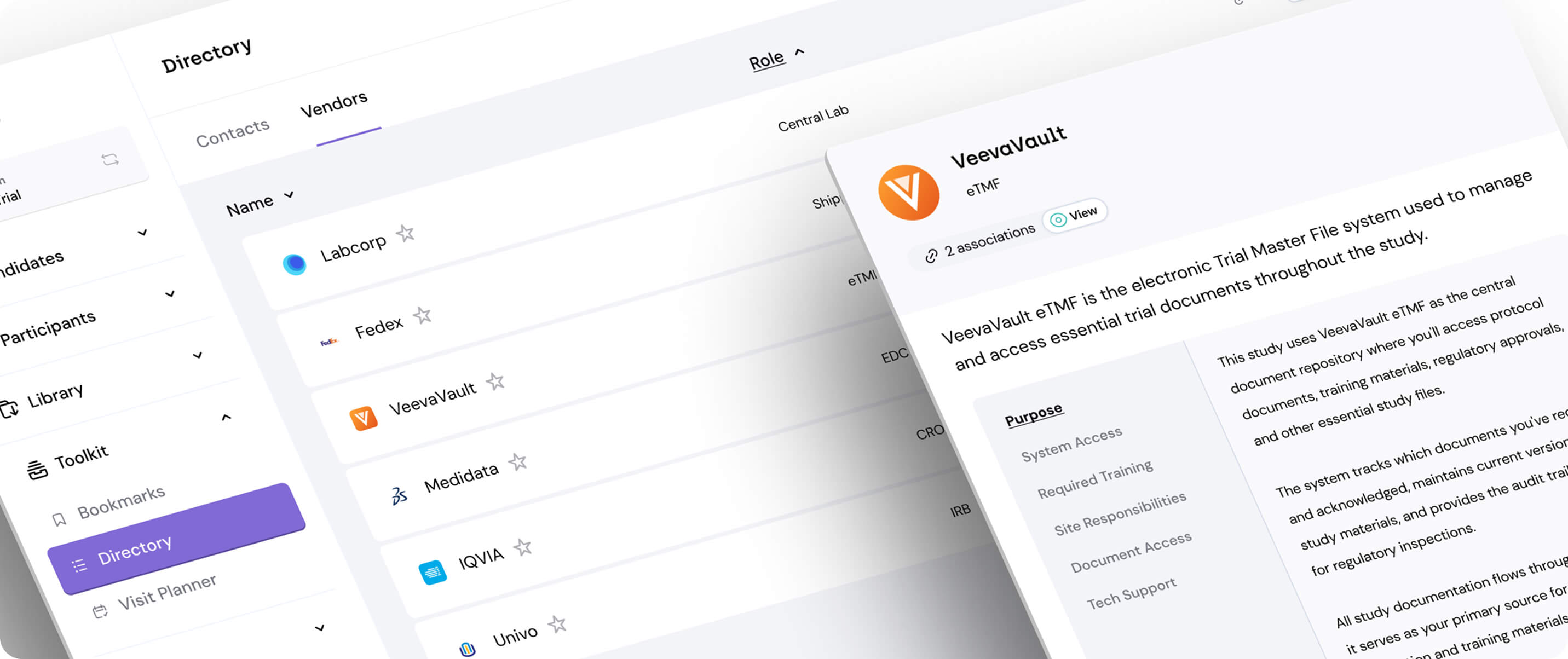
Relay is designed to feel like an extension of your sponsor team - and brand customization is just the start
Stop feeling like a broken record by giving sites an easy way to find accurate guidance
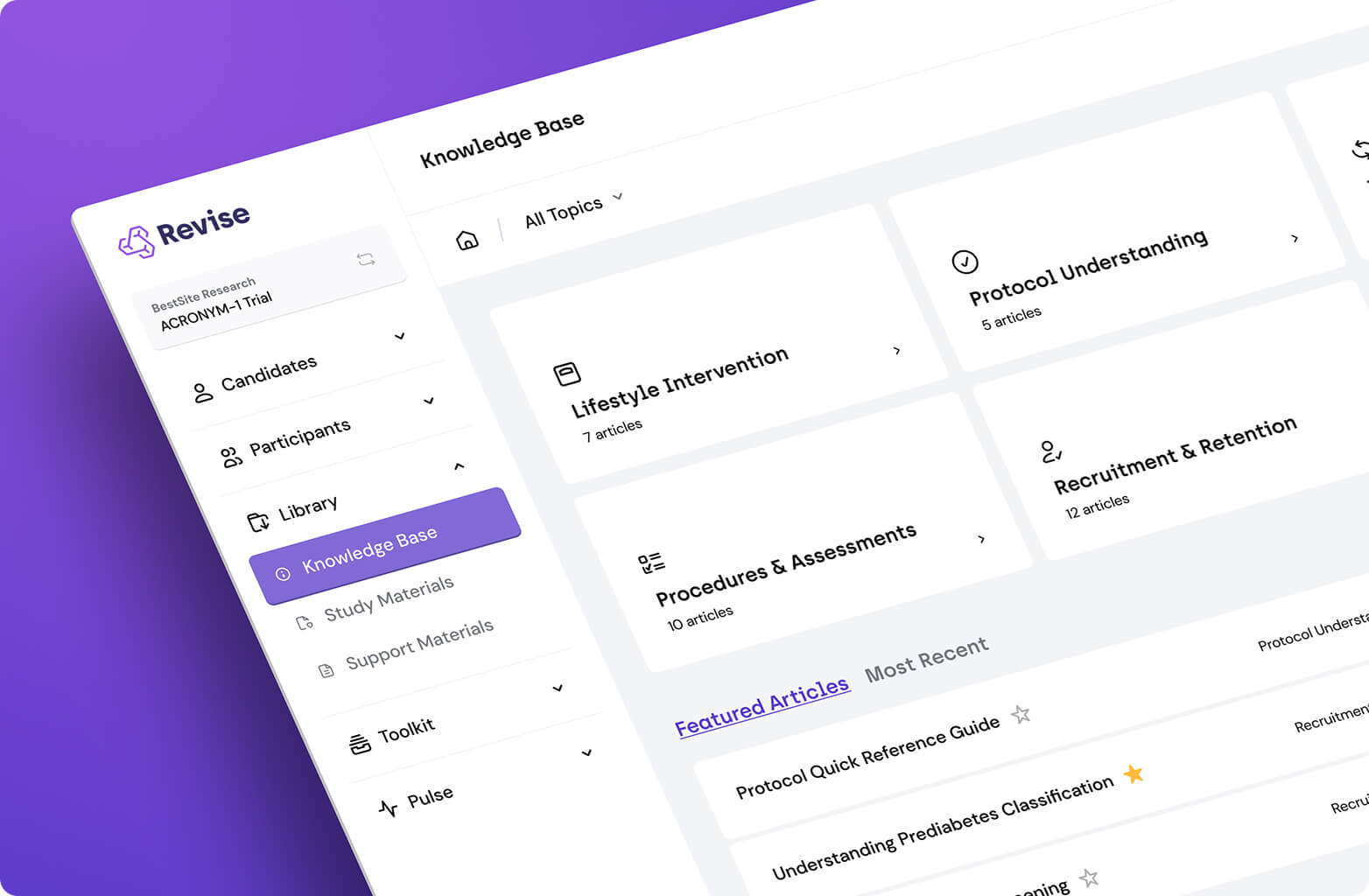
Turn visit chaos into clockwork with calculators, SoA detail, and more in a single site tool
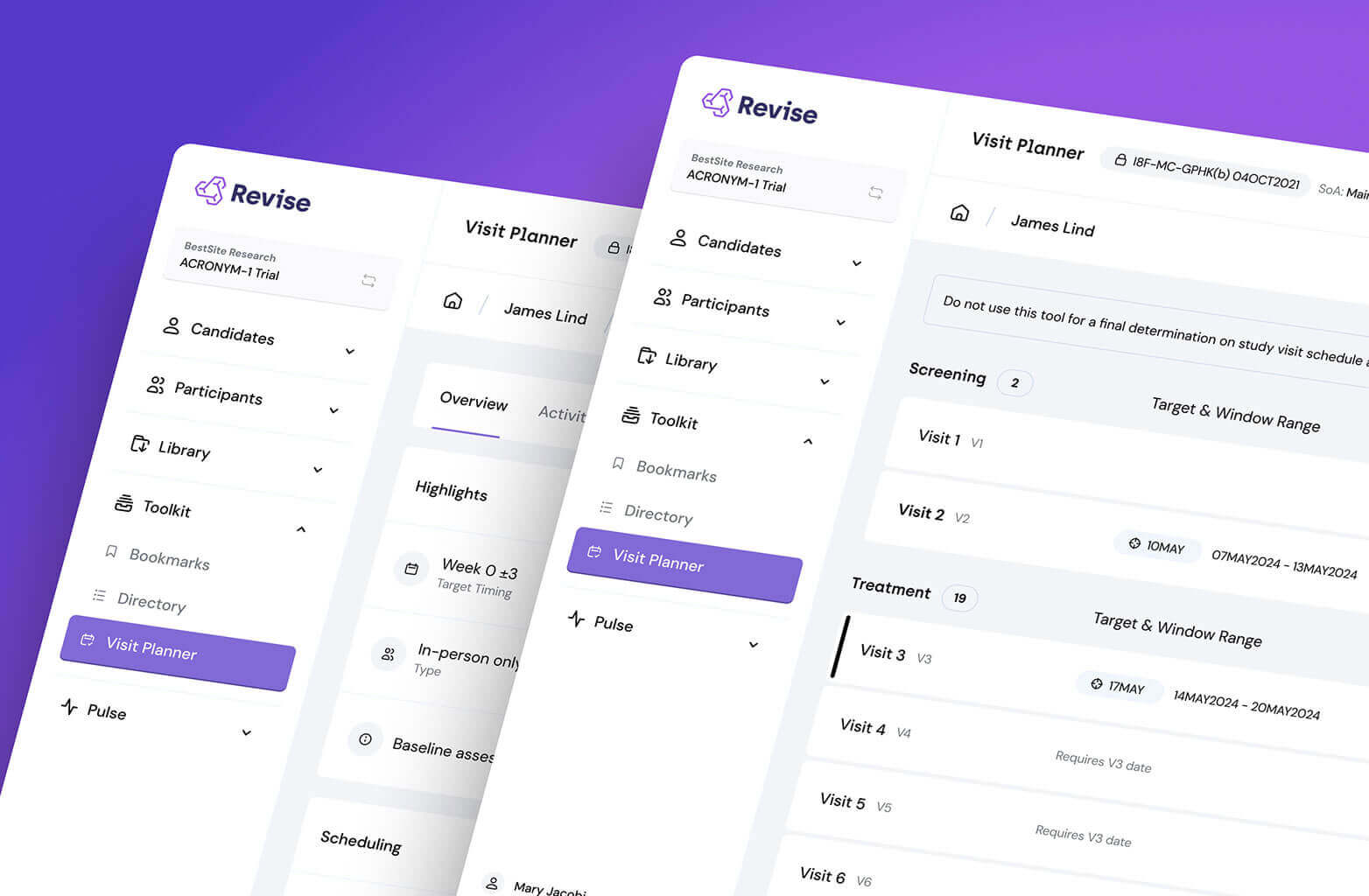
Tame tracking tedium while guiding sites through screening using a simple system

We worked with Revise because of the complexity of our program. Using Relay, we provided sites with the most recent version of study resources, as they were inevitably updated over the course of the study. Relay shines for long studies with varying stages over time, during which specific instructions or information need to be conveyed digitally.
Program Lead, Top 25 Pharma CompanyPush versions confidently knowing sites don't get access until they confirm IRB approval
Stop playing switchboard operator by giving sites key contact and vendor information
Grab limited site attention with bite-sized updates, and know if they've been seen
Help sites own their performance by giving them each a unique KPI dashboard
Stop overanalyzing anecdotes by taking a systematic approach to site feedback
Get to the ground truth of site activity with unfiltered data from their portal use
Prevent progress from stalling using site nudges based on smart thresholds - not nagging
Create confidence among candidates and sites by thinking bigger than checkboxes
Support retention and monitor dropout risk by getting a handle on key non-clinical data
Program Lead, Top 25 Pharma CompanyWorking with Revise to implement Relay helped us think through the lifecycle of the study in a way we had not previously considered. Previously, we focused mainly on FPV and early enrollment but not as much on later stages of the study and what information would be needed for retention. Revise was excellent in helping us think through this new way of communicating.
Deployed in 3 languages and 27 countries to distribute IRB materials to 700 sites in 43 patient languages on a 5-year trial
Site opt-in of 100% for Relay vs 12% for popular enrollment platform in head-to-head set up by sponsor on global oncology trial
Have more questions?
Maybe you shouldn’t. Everything you add to a study–from technology to people–increases operational complexity. Tech needs to be a strong solution to an important problem for it to be worth the tradeoff. Otherwise it just creates more problems.
The Relay platform addresses the problem of poor study communication between sponsors and sites. We've found this problem to be biggest on large, lengthy trials. Sometimes communication issues are obvious, but other times they show up in sneaky ways. For example, sponsor teams may find that they:
Get buried in manual processes and one-off conversations
Struggle to ensure sites keep key study info top of mind
Feel removed from sites, no matter what metrics they have
If none of this resonates, no need to read further. Relay is for sponsor teams who rate communication a high priority concern.
Now, for those still reading…you know what we are talking about. You know how many headaches poor study communication can cause, and you have major concerns about a current or future study.
In that case, check out the other FAQs and reach out if you want to learn more. We can tell you how we approach this problem and who tends to find Relay most valuable. But you know your needs best, and whether you should use our platform is not for us to say.
Sponsors have relied on Revise’s clinical trial and technology expertise for 15 years. We have a record of helping sponsors navigate their most complex clinical operations challenges, originally as Rebar Interactive and now as Revise. Teams look to us in a pinch. Here’s just one example:
During the height of COVID, a client texted our founder on a Sunday night. “Can you have a recruitment website live by Friday? The IRB has agreed to a 24-hour review.” We hit the deadline, and our client was so pleased that they used much of the website’s content and graphics in wider COVID communication.
We can deliver on these types of projects because we are funded by customers and backed by an agile, high-performing team of trial veterans. Each member of our core team has worked in clinical trials at least 18 years, whether at sites, CROs, or Revise. We are guided only by our expertise and the needs of those we serve, not investors.
Revise is also the only trial tech company led by a former research coordinator. Our founder has a deep appreciation for site frustration and a strong desire to alleviate it. This desire is why Revise was founded and is independently owned.
Our team’s experience is what led us to create the Relay platform. Relay began as a patient recruitment platform and was first deployed by top-20 pharma 7 years ago. We noticed that often sponsor challenges in recruitment and elsewhere were really about communication, so we evolved Relay to give sponsors a new approach.
Mostly no. You can continue to use preferred CROs and other vendors as much or little as you want, and however you want. We believe that this operational flexibility is important. We are not an “end-to-end” solution trying to be all things to all study teams. Nor is our aim to replace or consolidate vendors.
What we are trying to do is help sponsors regain ownership over site communication, especially on large trials. Sponsor teams have relied on vendors to communicate with sites because they have not had an easily scalable way to do it themselves. Relay gives them a way.
Relay establishes a dedicated channel for you to communicate with sites that remains independent of vendor mix and works on studies of any size. Your vendors can focus on providing the capability you hired them for, not being an intermediary. And you can focus on maintaining strong, direct communication with sites.
So the change is that you won’t communicate with sites through vendors as much. If your team prefers to be hands off with site communication, then Relay will not be a good fit. But if your team wants more ownership of site communication and isn’t sure how to make it happen, Relay is for you.
Sponsor study teams who work with us tend to have these qualities:
Have a trial with several hundred sites, often spanning multiple years
Want their internal study team to have more direct impact at the site level
Would like their own separate, scalable communication path with sites
Find that more headcount is not getting the results they want
Have emerging needs that their usual vendors are not able to meet
Our clients also often appreciate that we:
Can be more agile and responsive than large and investor-backed companies
Act as a knowledgeable partner, or what clients have called "the easy button"
Care most about easing the work of study teams, not industry disruption
Design tech with the belief that a strong site experience is the key to success
We will not be a good fit if you:
Have no major concerns about your existing study communication methods
Prefer that all site communication be filtered through CROs or other vendors
Prioritize feature quantity over quality, or are seeking an “end-to-end” solution
Only want tech in established categories (EDC, eTMF, eConsent, ePro/eCOA, etc.)
Associate investor backing, large teams, or high market visibility with competence
We may also not be a good fit if your study is less than a couple hundred sites. Relay is designed to work well at any scale, but sponsors tend to find more value as trials increase in number of sites and years. That’s when standard communication methods really start to break down and an alternative approach becomes most critical.